Behavior-Based Pricing™
Delivering evidence-based pricing and market access strategies to the pharmaceutical industry
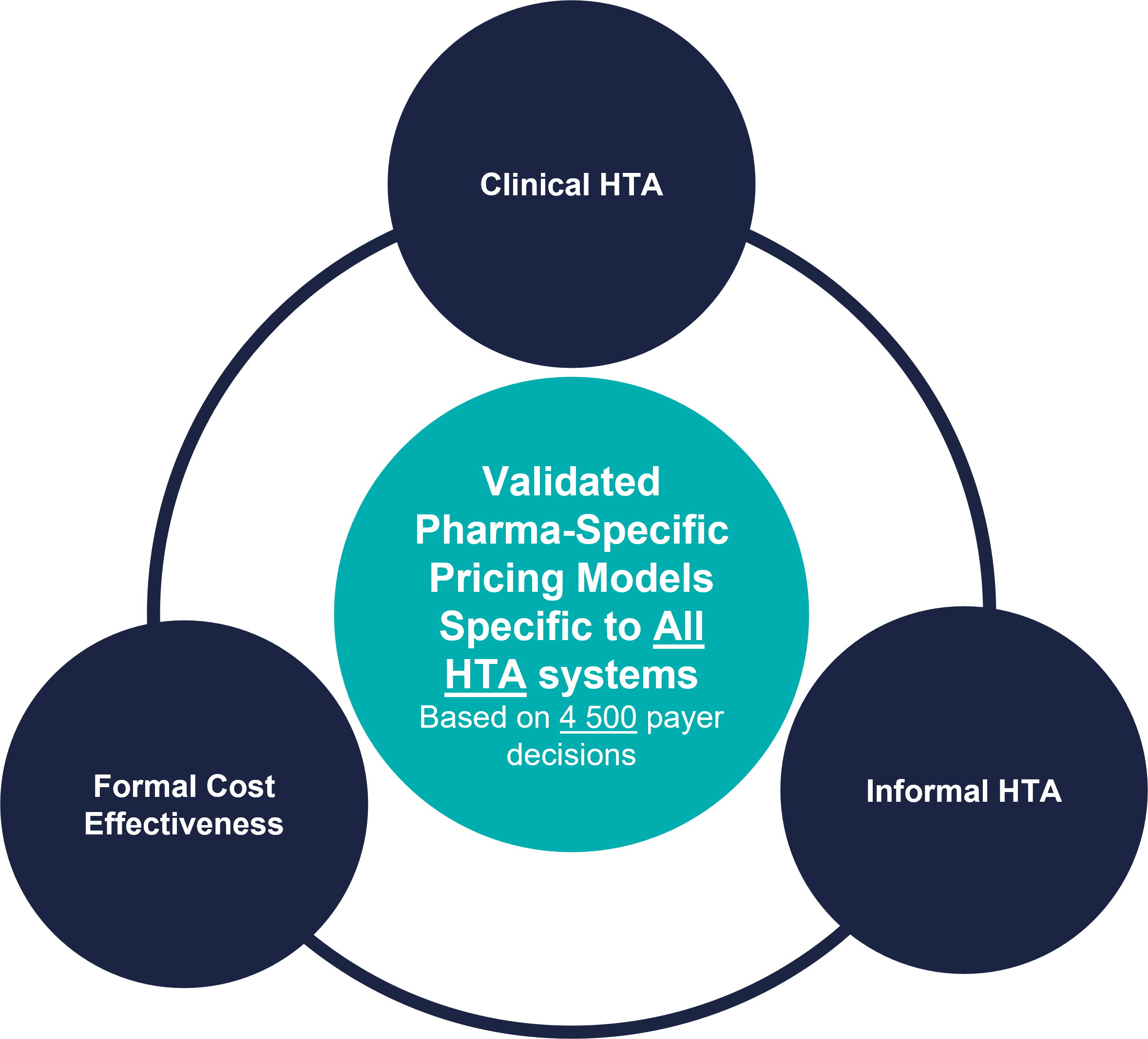
Behavior-Based Pricing applies to all HTA systems
Predicting the market access and reimbursement prospects for a pipeline asset requires both an understanding of the HTA and pricing systems in place in the target country and the past behavior of payers. Inpharmation’s Behavior-Based Pricing platform provides validated pricing and market access models that:
- Combine analytical tools that are both specific to country pricing systems and built upon thousands of real-world pharmaceutical pricing decisions.
- Provide market access insights with up to 93% predictive validity.*
- Draw upon insights from more than 4 500 historical payer decisions.
*Predictive validity from hold-out tests based upon real-world pharma reimbursement decisions
Predictive validity from hold-out tests based upon real-world pharma reimbursement decisions.
Inpharmation’s Behavior-Based Pricing platform provides pharma-validated pricing and market access insights specific to the pricing system in your research countries. Specific models provide insights for the following types of pricing systems:
- Clinical HTA pricing systems: e.g. Germany. In countries with clinical HTA pricing systems, drugs are rated according to the level of clinical advance that they represent. Pricing prospects are driven by that rating. The Behavior-Based Pricing platform allows you to estimate the level of advance that your asset promises and hence its pricing and market access prospects under this type of system.
- Formal cost-effectiveness systems: e.g. the UK. Countries with formal cost-effectiveness systems assess the cost-effectiveness of drugs according to a set health-economic methodology. The Behavior-Based Pricing platform allows you to rapidly estimate the cost-effectiveness of your asset (using battle tested systems like QALY-Mapping™ and Cost Mapping™ – and hence its pricing prospects – under such a system.
- Informal HTA systems: e.g. US health plans. Countries with informal HTA systems may review drugs very thoroughly, but there is usually no formally laid out pathway to determine pricing prospects. Instead, higher prices tend to require higher levels of effectiveness and supporting evidence, but there is no prescribed formula. The Behavior-Based Pricing platform uses the de facto rules (using technologies such as Exclusion Charts) that are revealed by examining large volumes of actual behavior (decisions) in such systems.
Of the top-10 global pharma companies use Inpharmation’s Behavior-Based Pricing solution.
"Europe's most respected pharma forecasting & pricing specialist consultancy."
Centre for Executive Leadership
Behavior-Based Pricing is an evidence-based approach to pharmaceutical pricing
You have only one opportunity to set your pharmaceutical pricing and market access strategy; that’s why Inpharmation recommend evidence-based pricing approaches.
Traditional analogue-based pricing approaches cannot offer you the high level of predictive validity (93%*) of Inpharmation’s econometric modelling. The reason is straightforward when you think about it; if you pick the wrong analogue for your pricing and market access strategy, then your probability of success is drastically reduced.
If you build your pricing and market access strategy on a solid evidence-base of over 4 500 historical payer decisions, taking account of the 7 most important drivers of pharma brand price potential, your market access insights could be truly transformed.
*Predictive validity from hold-out tests based upon real-world pharma reimbursement decisions

The evidence behind Inpharmation’s fact-based approach to pricing is meticulously documented in the book Value Pricing for Market Access: Evidence-Based Pricing for Pharmaceuticals by Inpharmation’s founder and Chairman, Gary Johnson.